| Revised on April 1st 2004 | ||||||||||||||||||||
|
Cell Adhesion, Extracellular Matrix and Epithelial Morphogenesis |
||||||||||||||||||||
|
(This article
is produced with modifications from the original paper by Hieda and
Nakanishi published in Develop. Growth & Differ.,39, 1-8, 1997.) (Related papers) |
||||||||||||||||||||
| Introduction | ||||||||||||||||||||
|
Embryonic
rudiments of most organs including lung, kidney, submandibular gland,
hair and mammary gland are composed of two types of tissues, the epithelium
and the mesenchyme, and these two tissues are separated by a basal
lamina. Growing epithelial tissue changes and acquires a shape unique
to each organ during development. The epithelial shape change is well
known to require tissue interactions with the surrounding mesenchyme.
It is one of the major issues in developmental biology to understand
how shape change of the epithelial tissue is achieved and what is
the nature of the tissue interactions between epithelium and mesenchyme. The mouse embryonic submandibular gland has been used as a model system for analyzing the mechanisms of epithelial shape change and of the epithelial-mesenchymal interactions (Borghese, 1950; Grobstein, 1953). The organ rudiment can be cultured on a membrane filter or agar, and the epithelial shape change is easily followed by light microscopy. In the organ culture system, the effects of various reagents on the morphogenesis can be tested. Furthermore, the epithelium and mesenchyme can be separated from each other by treatment with collagenase, dispase or other proteases, making various recombination experiments possible. Formation of the mouse submandibular gland begins on late day 11 of gestation. The initial epithelial primordium arises from the invagination of the oral epithelium into the mandibular mesenchyme and then undergoes extensive branching morphogenesis during its development. |
||||||||||||||||||||
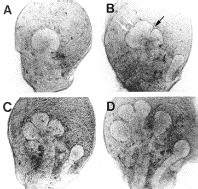
|
||||||||||||||||||||
|
At the 12-day stage a bulbous
lobule is formed at the distal region of a stalk (A) and later, several
indentations appear on surface of the epithelial lobule (B) and subsequently
a couple of them become narrow and deep clefts (C). Widening of the
clefts and epithelial expansion lead to branching of the lobule into
smaller ones by the late 13-day stage (D). After repetitive branching
morphogenesis, the shape of the epithelium becomes like a bunch of
grapes. Concomitantly with the branching morphogenesis, the epithelium also exhibits change in tissue architecture (Redman, 1987). The epithelial primordium derived from the oral epithelial sheet is formed as a mass of cells and not as a cell sheet. After substantial branching morphogenesis occurs in this cell mass, definite lumens begin to form in the branched epithelium at the late 14-day stage, leading to a fully cavitated epithelial tissue. It has been suggested that epithelial activities such as cell proliferation (Bernfield, Banerjee & Cohn, 1972; Bernfield & Banerjee, 1982; Lawson, 1974) and co-ordinated changes in cell shape (Spooner, 1973; Spooner & Wessells, 1972; Ash, Spooner & Wessells, 1973) may drive branching morphogenesis of the epithelium. In this context, extensive studies have been made on regulatory roles of mesenchymal factors and the extracellular matrix such as interstitial collagens and heparan sulfate proteoglycan, including the basal lamina, in the epithelial morphogenesis. On the other hand, it has been revealed that the submandibular mesenchyme itself also exhibits dynamic morphogenetic movement (click here for an animation, in preparation) and that the mesenchymal cells can generate physical force in cooperation with the extracellular matrix (Nogawa, 1983; Nogawa & Nakanishi, 1987). These observations raise the possibility that the mesenchyme plays mechanical roles in shape change in epithelial tissue (click here for the hypothesis). In an effort to give a mechanistic view of early epithelial shape changes in the developing submandibular gland, in this article, we will mainly focus on the morphogenetic activities of the submandibular mesenchyme and its regulation of epithelial architecture, to which little attention has been paid. |
||||||||||||||||||||
| Morphogenetic activities of the submandibular mesenchyme | ||||||||||||||||||||

|
||||||||||||||||||||

|
||||||||||||||||||||
|
||||||||||||||||||||
|
Inhibition by collagenase
and stimulation by collagenase inhibitor Among various extracellular matrix components present in submandibular glands (Hardman & Spooner, 1992a), involvement of collagens in epithelial branching morphogenesis has been well established. Inhibitors of collagen synthesis and secretion, L-azetidine-2-carboxylic acid and a,aユ-dipyridyl (Spooner & Faubion, 1980), as well as commercially available bacterial collagenase preparations (Grobstein & Cohen, 1965), have been shown to inhibit branching morphogenesis of epithelium. Although L-azetidine-2-carboxylic acid affected the synthesis of other proteins to some degree and the collagenase preparations possibly contained glycosaminoglycan-degrading enzymes and proteases (Bernfield, Banerjee and Cohn, 1972), a highly-purifiedClostridialcollagenase, which degrades all types of collagens but not other proteins, has been shown to inhibit not only the initiation of cleft formation in epitheium of the 12-day gland but also further epithelial branching in the 13-day gland (Nakanishi et al.,1985; Nakanishi et al., 1986a; Nakanishi et al., 1986b). Furthermore, a collagenase from bovine dental pulp, which degrades collagens I and III but not collagens IV and V, has been shown to inhibit cleft formation in the epithelium of 12-day submandibular glands (Fukuda et al., 1988). This indicates the involvement of interstitial collagens present in the mesenchyme in the initiation of cleft formation. In contrast, a collagenase inhibitor purified from the culture medium of bovine dental pulp cells, which is a tissue inhibitor of metalloproteases, promoted the formation of more clefts than were formed in control glands (Nakanishi et al., 1986a, b), suggesting the causative role of collagen in cleft formation. |
||||||||||||||||||||
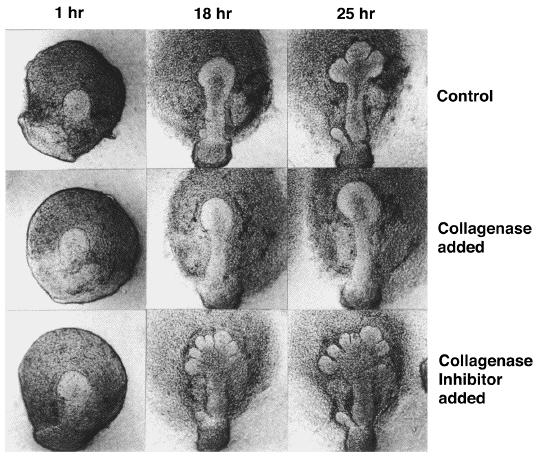 (Scott F. Gilbert, "Developmental Biology" 5th Edition, pp. 684-686, 1997 and Y. Nakanishi et al., Developmental Biology, 113, 201-206, 1986 ) |
||||||||||||||||||||

|
||||||||||||||||||||
|
||||||||||||||||||||
|
Electron microscopic observations have shown that the basal
lamina at the distal part of the lobule is thin and discontinuous
while that in the wide clefts and in the stalk region is rather thick
and continuous (Coughlin, 1975; Cohn et al., 1977; Spooner and Faubion,
1980; Fukuda et al., 1988). In addition, the rate of turnover of glycosaminoglycans
in the basal lamina has been reported to be faster at the tip of the
lobule than at the wide clefts (Bernfield & Banerjee, 1982). Thus,
stabilization of the basal lamina in the wide clefts and in the stalk
region is suggested to be involved in epithelial morphogenesis. In
relation to the stability of the basal lamina, it has been suggested
that remodeling of the basal lamina is associated with epithelial
cell proliferation, a suggestion based on the observation that the
proliferation activity of the epithelium was high in the distal region
of lobules compared with that in the wide clefts (Bernfield & Banerjee,
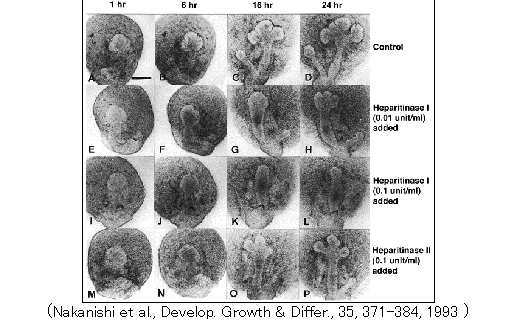
1982). It has been shown that heparan sulfate proteoglycan binds to fibers of interstitial collagens under physiological conditions (Koda et al., 1985); that heparitinase, which inhibits epithelial morphogenesis, brings about the dissociation of collagen fibers from the basal lamina (Nakanishi et al., 1993); and that antibodies against domain E3, a heparin-binding site, of laminin-1 perturb epithelial morphogenesis (Kadoya et al., 1995). It is likely that interactions among collagen networks in the mesenchyme and laminin and heparan sulfate proteoglycans in the basal lamina are required for branching morphogenesis of the epithelium (Nakanishi et al., 1993). |
||||||||||||||||||||

|
||||||||||||||||||||

|
||||||||||||||||||||
| Regulation of epithelial architecture and cell adhesion systems by mesenchyme | ||||||||||||||||||||
|
Development of
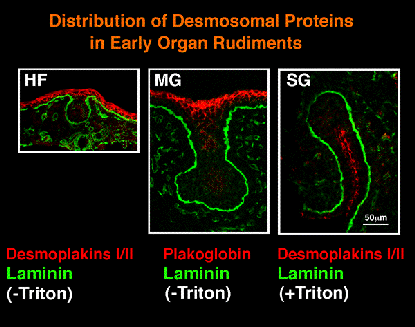
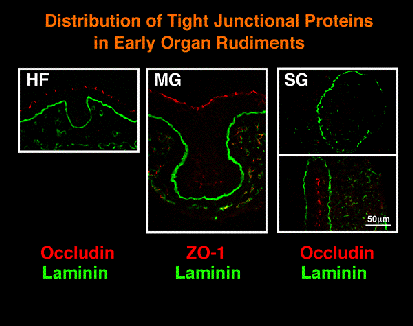
epithelial architecture and cell-cell adhesion systems As described earlier, epithelial morphogenesis in submandibular gland involves a change in the tissue architecture from a mass of cells to a cavitated sheet. It has been shown that this architectural change in the epithelium is accompanied by drastic changes in the organization of cell-cell adhesion systems. Previous electron microscopic observations have shown that cell-cell junctions including adherens junctions, desmosomes, and tight junctions are rarely seen in the early epithelial tissue making a cell mass but become organized later (Redman, 1987; Kadoya and Yamashina, 1993). These observations have been confirmed and extended by a recent study employing immunofluorescence microscopy with antibodies against components of the cell-cell adhesion systems (Hieda et al., 1996). |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
In the 12-day and 13-day stages, desmoplakins
I/II (major desmosomal proteins) and occludin and ZO-1 (tight junctional
proteins), but not the E-cadherin/b-catenin complex, were hardly detected
in the epithelial lobules while all these molecules were present in
the stalk region and in the oral epithelium. Similar situations were
observed in the developing rudiments of hair follicles and mammary
gland. It is of note that the E-cadherin/b-catenin complex and the
associated microfilaments were uniformly distributed along the cell
periphery in the lobules, indicating that adherens junctions had not
yet formed. In the 14-day glands, expression of these molecules associated
with cell-cell adhesion systems became evident especially in the forming
cavitated regions. These data indicate that cell-cell adhesion systems
are less developed in the early epithelial lobules packed with cells
and that there is a close relationship between changes in tissue architecture
of the epithelium and those in the organization of cell-cell adhesion
systems during the development. Regulation by tissue interactions with mesenchyme Our recent recombination experiments using submanidbular gland and lung rudiments have shown that submandibular mesenchyme plays important roles in the organization of the tissue architecture and cell-cell adhesion systems in the epithelium (Iwai et al., Develop, Growth & Differ, 1998). Lung mesenchyme was able to maintain both the organization of the lung epithelium, in which an epithelial sheet of columnar cells lines a relatively large lumen, and the apico-lateral distribtution of E-cadherin in the cells. However, when lung epithelium was cultured with the submandibular mesenchyme, the regular epithelial organization was disturbed, and E-cadherin lost its apical distribution and became re-distributed to the baso-lateral region of the cells. Little information is available on the molecular mechanisms of the mesenchymal regulation. Novel distribution patterns revealed by immunofluorescence microscopy, however, suggest the possible involvement of epimorphin, which is a mesenchymal factor required for epithelial morphogenesis and for maintenance of cell adhesion systems and polarity of epithelial cells (Hirai et al., 1992). In submandibular glands, epimorphin distribution was hardly detected at the 12-day stage but became evident in the mesenchyme and the basal lamina region at the 13-day stage (Kadoya et al., 1995; Nanba et al., unpublished data, 1998). Noticeably, accumulation of epimorphin around the basal lamina was restricted to the stalk and not observed in the lobular region (Nanba et al., unpublished data, 1996). The appearance of the less-organized submandibular epithelium appears to involve regulation of the epimorphin deposition at the basal lamina. The lack of apparent inhibition of the submandibular gland morphogenesis by a monoclonal antibody against epimorphin may be due to the absence of epimorphin from the lobular region exhibiting active morphogenesis (Kadoya et al., 1995; Nakanishi, unpublished data, 1993). Recently the E-cadherin distribution in submandibular epithelium has been shown to be affected by a monoclonal antibody against the integrin a6 subunit, which is distributed at the basal surface of the epithelium (Kadoya et al., 1995). The antibody inhibited the branching morphogenesis to some extent and brought about loss of the E-cadherin distribution in the inner but not peripheral area of epithelial lobules packed with cells. Since integrin a6b1 recognizes domain E8 of laminin-1 (Hall et al, 1990), constitutive expression of E-cadherin in the epithelial lobules may require signals due to interactions of epithelial cells with laminin-1, which is specifically localized in the basal lamina (Kadoya et al., 1995). |
||||||||||||||||||||
| Conclusion | ||||||||||||||||||||
|
It has been well known that epithelial branching morphogenesis during
the early development of the mouse embryonic submandibular gland requires
tissue interactions with the surrounding mesenchyme. The mesenchymal
cell population shows active flowing movement and can generate physical
forces in cooperation with collagen fibers, while the epithelial organization
is likely to become loose because of down-regulation of intercellular
adhesion systems, as described above. Changes in the epithelial contour
would therefore be dependent on such tissue properties of the epithelium
and the mesenchyme, which are kept in contact in intact organ. Some previous studies have attributed the formation of clefts to the epithelial cell proliferation and to co-ordinated contraction of actin-containing microfilaments leading to epithelial shape changes (Hardman & Spooner, 1992b). However, cleft formation seems to be much more complex, involving dynamics of tissue organization of both the mesenchyme and the epithelium. We have proposed a model in which clefts on epithelial surfaces are formed by mesenchymal forces that result from the ability of the moving mesenchymal cells to contract collagen fibers and to promote the alignment of these fibers (Nakanishi et al., 1986c; Nakanishi & Ishii, 1989).The model could be extended by taking into consideration the following notion concerning responses of cell aggregates to external forces: When external forces are applied, cell aggregates initially respond as elastic bodies with internal tensions, and if the forces persist, the internal tensions dissipate due to the ability of the cells to slip past one another (Steinberg, 1978; Foty et al., 1996). As has been reported, before definite clefts are formed on early epithelial lobules of the submandibular gland, many tiny indentations appear and disappear on the epithelial surfaces (Nogawa, 1983; Hieda and Nakanishi, unpublished data). When the mesenchymal forces exerted on the epithelial surfaces are transient, probably due to the instability of nascent collagen bundles and of their interactions with the mesenchymal cells, such unstable indentations will occur if the epithelium has elastic properties. The elastic responses of the early epithelial tissue during initial cleft formation is also suggested by the observations that shallow but not deep clefts disappear after collagenase treament of the whole glands or the removal of the mesenchyme by protease treatment. However, as the submandibular gland development proceeds, the mesenchymal forces become persistent at certain sites of the epithelial surfaces, probably because of stable supracellular complexes containing collagen bundles and mesenchymal cells. This may be accompanied by dissipation of internal tensions because of the ability of cells to slip past one another which ability could be facilitated by the down-regulation of intercellular adhesion systems (Hieda et al., 1996). This, in turn, would help shallow clefts deepen more into the epithelium, and increasing deposition of various types of collagens including the basal lamina network would make the clefts more stable. Even if the contraction of actin-containing microfilaments of basal epithelial cells is directly related to the cleft formation as had been proposed, the properties of the early epithelial lobules with loose cell-cell adhesion systems would be an essential prerequisite for stable cleft formation. Finally, it is noteworthy that cell adhesion systems are also less developed in early epithelial buds of hair follicle and mammary gland (Nanba et al., submitted) and in the inner cell mass in early mouse embryos (Fleming et al., 1994). Furthermore, accumulation of epimorphin at the basal lamina region is not observed aroung the early epithelial buds of hair follicle and mammary gland (Nanba et al., submitted). These observations suggest that common mechanisms work during formation of early epithelial tissues in various organ rudiments. References Ash, J. F., B. S. Spooner and N. K. Wessells. 1973. Effects of papaverine and calcium-free medium in salivary gland morphogenesis. Dev. Biol.33, 463-469. Banerjee, S. D., R. H. Cohn and M. R. Bernfield. 1977. Basal lamina of embryonic salivary epithelia. Production by the epithelium and role in maintaining lobular morphology. J. Cell Biol. 73, 445-463. Bell, E., B., Ivarsson and C., Merrill. 1979. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potentialin vitro. Proc. Natl. Acad. Sci. U.S.A. 76, 1274-1278. Bernfield, M. and S. D. Banerjee. 1972. Acid mucopolysaccharaide (glycosaminoglycan) at the epithelial-mesenchymal interface of mouse embryo salivary glands. J. Cell Biol. 52, 664-673. Bernfield, M., S. D. Banerjee and R. H. Cohn. 1972. Dependence of salivary epithelial morphology and branching morphogenesis upon acid mucopolysaccharide-protein (proteoglycan) at the epithelial surface. J. Cell Biol.52, 674-689. Bernfield, M. and S. D. Banerjee. 1982. The turnover of basal lamina glycosaminoglycan correlates with epithelial morphogenesis. Dev. Biol.90, 291-305. Borghese, E. 1950. The developmentin vitroof the submndibular and sublingual glands ofMus musculus. J. Anat.84, 287-302. Cohn, R. H., S. D. Banerjee and M. R. Bernfield. 1977. Basal lamina of embryonic salivary epithelia. Nature of glycosaminoglycan and organization of extracellular materials. J. Cell Biol.73, 464-478. Coughlin, M. D. 1975. Early development of parasympathetic nerves in the mouse submandibular gland. Dev. Biol.43, 123-139. Fleming, T. P., L. Butler, X. Lei, J. Collins, Q. Javed, B. Sheth, N. Stoddart, A. Wild and M. Hay. 1994. Molecular maturation of cell adhesion systems during mouse early development. Histochem.101, 1-7. Foty, R. A., C. M. Pfleger, G. Forgacs and M. S. Steinberg. 1996. Surface tensions of embryonic tissues predict their mutual envelopment behavior. Development122, 1611-1620. Fukuda, Y., Y. Masuda, J. Kishi, Y. Hashimoto, T. Hayakawa, H. Nogawa and Y. Nakanishi. 1988. The role of interstitial collagens in cleft formation of mouse embryonic subamandibular gland during initial branching. Development103, 259-267. Grobstein, C. 1953. Epithelio-mesenchymal specificity in the morphogenesis of mouse sub-mandibular rudiments in vitro. J. Exp. Zool.124, 383-413. Grobstein, C. and J. Cohen. 1965. Collagenase: Effect on the morphogenesis of embryonic salivary epithelium in vitro. Science150, 626-628. Hall, D. H., L. F. Reichardt, E. Crowley, B. Holley, H. Moeaai, A. Sonnenberg and C. H. Damsky. 1990. The a1/b1 and a6/b1 integrin heterodimers mediate cell attachment to distinct sites of laminin. J. Cell Biol.110, 2175-2184. Hardman, P. and B. S. Spooner. 1992a. Localization of extracellular matrix components in developing mouse salivary glands by confocal microscopy. Anat. Rec.234, 452-459. Hardman, P. and B. S. Spooner. 1992b. Salivary epithelium branching morphogenesis.InEpithelial Organization and Development (Ed. T. P. Fleming), pp. 353-375. Chapman & Hall, London. Harris, A. K., D. Stopak and P. Wild. 1981. Fibroblast traction as a mechanisms for collagen morphogenesis. Nature (London)290, 249-252. Hieda, Y., K. Iwai, T. Morita and Y. Nakanishi. 1996. Mouse embryoinc submandibular gland epithelium loses its tissue integrity during early branching morphogenesis. Dev. Dynamics,207, 395-403. Hirai, Y., K. Takabe, M. Takashima, S. Kobyashi and M. Takeichi. 1992. Epimorphin: A mesenchymal protein essential for epithleial morphogenesis. Cell69, 471-481. Ichikawa, K. and Y. Nakanishi. 1992. Three-dimensional computer images of the alignment of collagen III fibrils at epithelio-mesenchymal interfaces of the developing mouse submandibular gland. Acta Histochem. Cytochemica.26, 623-628. Iwai, K., Hieda, Y. and Nakanishi, Y. 1998. Effects of mesenchyme on epithelial tissue arichitecture revealed by tissue recombination experiments between submandibular gland and lung of embryonic mice. Develop. Growth & Differ., in press. Kadoya, Y. and S. Yamashina. 1993. Distribution of a6 integrin subunit in developing mouse submandibular gland. J. Histochem. Cytochem.41, 1707-1714. Kadoya, Y., K. Kadoya, M. Durbeej, K. Holmvall, L. Sorokin and P. Ekblom. 1995. Antibodies against domain E3 of laminin-1 and integrin a6 subunit perturb branching morphogenesis of submandibular gland, but by different modes. J. Cell Biol.129, 521-534. Koda, J. E., A. C. Rapraeger and M. Bernfield. 1985. Heparan sulfate proteoglycans from mouse mammary epithelial cells: cell surface proteoglycan as a receptor for interstitial collagens. J. Biol. Chem.260, 8156-8162. Kratochwil, K., M. Dziadek, J. Lohler, K. Harbers and R. Jaenisch. 1986. Normal epithelial branching morphogenesis in the absence of collagen I. Dev. Biol.117, 595-606. Lawson, K. A. 1974. Mesenchymal specificity in rodent salivary gland development: the response of salivary epithelium to lung mesenchymein vitro. J. Embryol. exp. Morph.32, 469-493. Montesano, R., K. Matsumoto, T. Nakamura and L. Orci. 1991. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell67, 901-908. Mori, Y., K. Yoshida, T. Morita and Y. Nakanishi. 1994. Branching morphogenesis of mouse embryonic submandibular epithelia cultured under three different conditions. Devop. Growth & Differ.36, 529-539. Mori, Y., Y. Hieda, T. Morita and Y. Nakanishi. 1995. Salivary gland morphogenesis: Involvement of extraellular matrix components during the dynamic processes.InInterplay of Genetic and Physical Processes in the Development of Biological Form (Ed. D. Beysens, G. Forgacs and F. Gaill), pp. 113-120. World Scientific Publishing, Singapore. Nakanishi, Y., F. Sugiura, J. Kishi and T. Hayakawa. 1985. Tissue collagenase regulates branching morphogenesis of mouse embryonic salivary gland.InBasement Membranes (Ed. S. Shibata), pp. 441-442. Elsevier Science Publishers B. V., Amsterdam, The Netherlands. Nakanishi, Y. and T. Ishii. 1989. Epithelial shape change in mouse embryonic submandibular gland: modulation by extracellular matrix components. BioEssays11, 163-167. Nakanishi, Y., F. Sugiura, J. Kishi and T. Hayakawa. 1986a. Collagenase inhibitor stimulates cleft formation during early morphogenesis of mouse salivary gland. Dev. Biol.113, 201-206. Nakanishi, Y., F. Sugiura, J. Kishi and T. Hayakawa. 1986b. Local effects of implanted Elvax chips containing collagenase inhibitor and bacterial collagenase on branching morphogenesis of mouse embryonic submandibular glandsin vitro. Zool. Sci.3, 479-486. Nakanishi, Y., F. Sugiura, J. Kishi and T. Hayakawa. 1986c. Scanning electron microscopic observation of mouse embryonic submandibular glands during initial branching: preferential localization of fibrillar structures at the mesenchymal ridges participating in cleft formation. J. Embryol. exp. Morph.96. 65-77. Nakanishi, Y., T. Morita and H. Nogawa. 1987. Cell proliferation is not required for the initiation of early cleft formation in mouse embryonic submandibular epitheliumin vitro. Development99, 429-437. Nakanishi, Y., H. Nogawa, Y. Hashimoto, J. Kishi and T. Hayakawa. 1988. Accumulation of collagen III at the cleft points of developing mouse submandibular epithelium. Development104, 51-59. Nakanishi, Y., J. Uematsu, H. Takamatsu, Y. Fukuda and K. Yoshida. 1993. Removal of heparan sulfate chains halted epithelial branching morphogenesis of the developing mouse submandibular glandin vitro. Develop. Growth & Differ.35, 371-384. Nogawa, H. 1983. Determination of the curvature of epithelial cell mass by mesenchyme in branching morphogenesis of mouse salivary gland. J. Embryol. exp. Morph.73, 221-232. Nogawa, H. and Y. Nakanishi. 1987. Mechanical aspects of the mesenchymal influence on epithelial branching morphogenesis of mouse salivary gland. Development101, 491-500. Nogawa, H. and Y. Takahashi. 1991. Branching morphogenesis of mouse salivary epithelium in basement membrane-like substratum separated from mesenchyme by the membrane filter. Development111, 327-335. Redman, R. S. 1987. Development of the salivary glands.InThe Salivary System (Ed. L. M. Sreebny), pp. 1-20, CRC Press, Florida. Spooner, B. S. 1973. Microfilaments, cell shape changes and morphogenesis of salivary epithelium. Amer. Zool.13, 1007-1022. Spooner, B. S. and N. K. Wessells. 1972. An analysis of salivary gland morphogenesis: Role of cytoplasmic microfilaments and microtubules. Dev. Biol.27, 38-54. Spooner, B. S. and J. M. Faubion. 1980. Collagen involvement in branching morphogenesis of embryonic lung and salivary gland. Dev. Biol.77, 84-102. Spooner, B. S., K. Bassett and B. Stokes. 1985. Sulfated glycosaminoglycan deposition and processing at the basal epithelial surface in branching and b-D-xyloside-inhibited embryonic salivary glands. Dev. Biol.109, 177-183. Spooner, B. S., K. E. Bassett and B. S. Spooner, Jr. 1989. Embryonic salivary gland epithelial branching activity is experimentally independent of epithelial expansion activity. Dev. Biol.133, 569-575. Steinberg, M. S. 1978. Cell-cell recognition in multicellular assembly: levels of specificity.InCell-Cell Recognition S.E.B. Symposium, No. 32 (Ed. A. S. G. Curtis), pp. 583-607. Cambridge, England: Cambridge University Press. Steinberg, B. M., K. Smith, M. Colozzo and R. Pollack. 1980. Establishment and transformation diminish the ability of fibroblasts to contract a native collagen gel. J. Cell Biol.87, 304-308. Stopak, D. and A. K. Harris. 1982. Connective tissue morphogenesis by fibroblast traction. I. Tissue culture observations. Dev. Biol.90, 383-398. Takahashi, Y. and H. Nogawa. 1991. Substitution for mesenchyme by basement membrane-like substratum and epidermal growth factor in inducing branching morphogenesis of mouse salivary epithelium. Development112, 855-861. Thompson, H. A. and B. S. Spooner. 1982. Inhibition of branching morphogenesis and alteration of glycosaminoglycan biosynthesis in salivary glands treated with b-D-xyloside. Dev. Biol.89, 417-424. Thompson, H. A. and B. S. Spooner. 1983. Proteoglycan and glycosaminoglycan synthesis in embryonic mouse salivary glands: Effects of b-D-xyloside, an inhibitor of branching morphogenesis. J. Cell Biol.96, 1443-1450. |
||||||||||||||||||||